Using boric acid gel for extraction of azaspiracids
Azaspiracids (AZAs) are marine algal toxins that can cause food poisoning in humans. Azaspiracids belong to a family of more than 50 polyether toxins originating from marine dinoflagellates such as Azadinium spinosum.
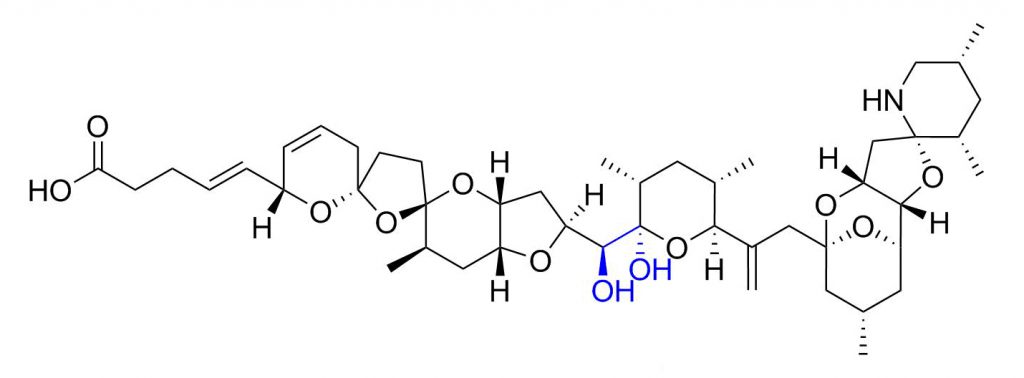
Figure 1: Structure of azaspiracid-1
Boric acid gel can bind selectively to compounds containing vic-diols or α-hydroxycarboxylic acids via formation of reversible boronate complexes. All of the azaspiracids reported thus far contain a 21,22-dihydroxy group. The aim was to check whether azaspiracids from blue mussel extracts – which is a complex matrix – could be selectively captured and released.

Figure 2: Blue mussel extract before applying it to the boric acid gel, and after the gel (load/wash) and three following elution fractions with acidified MeOH.
Analysis of the extracts and fractions by liquid chromatography−tandem mass spectrometry (LC−MS) showed that this procedure resulted in an excellent cleanup of the azaspiracids in the extract. Analysis by enzyme-linked immunosorbent assay (ELISA) and LC−MS indicated that most azaspiracid analogues were recovered in good yield by this procedure. The capacity of boric acid gel for azaspiracids was at least 50 μg/g, making this procedure suitable for use in the early stages of preparative purification of azaspiracids.
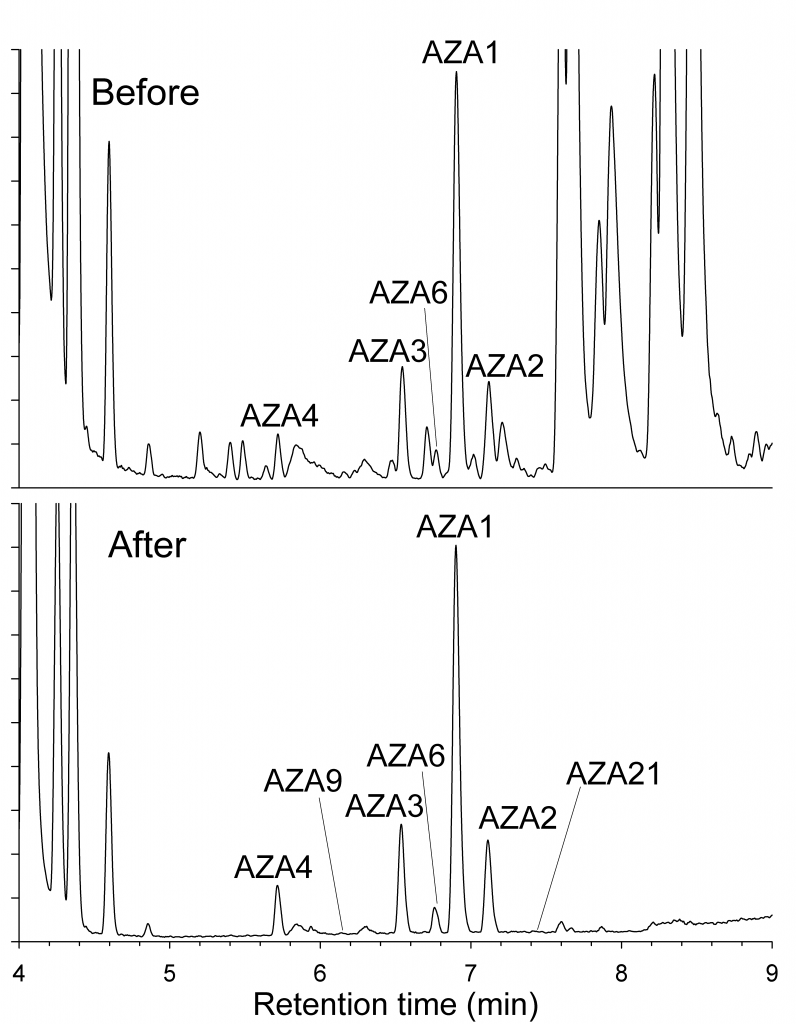
Figure 3: LC-MS-chromatogram of the shellfish sample before and after the boric acid gel column.
Table 1: Comparison of AZAs by ELISA and LC-MS in fractions from boric acid gel column fractionation of a shellfish sample.
ELISA (µg) LC-MS (µg) ratioapplied 20.9
load/wash 4.6 2.3 2.0
elute 1 25.9 16.9 1.5
elute 2 3.4 2.2 1.5elute 3 0.6 0.4 1.3
In addition to its potential for concentration of dilute samples, the extensive cleanup provided by boric acid gel fractionation of azaspiracids in mussel samples almost eliminated matrix effects during subsequent LC−MS and could be expected to reduce matrix effects during ELISA analysis. The method may therefore prove useful for quantitative analysis of azaspiracids as part of monitoring programs.
Although LC−MS data showed that okadaic acid analogues also bound to the gel, this was much less efficient than for azaspiracids under the conditions used. The boric acid gel methodology is potentially applicable to other important groups of natural toxins containing diols including ciguatoxins, palytoxins, pectenotoxins, tetrodotoxin, trichothecenes, and toxin glycosides.
For more information:
Miles CO, Kilcoyne J, McCarron P, Giddings SD, Waaler T, Rundberget T, Samdal IA, Løvberg KE. Selective Extraction and Purification of Azaspiracids from Blue Mussels (Mytilus edulis) Using Boric Acid Gel. J Agric Food Chem. 2018, xx, xxx-xxx.