The cyanobacterial toxins are a diverse group of natural toxins, both chemically and toxicologically. Cyanobacteria (also known as blue-green algae) produce a variety of unusual metabolites with unclear function, whereas some of this are toxic to humans and animals. During blooms of cyanobacteria, the cyanotoxins may accumulate in fish, crayfish or shellfish, causing food poisoning episodes.
The natural toxins include hepatotoxins (such as microcystins, nodularins and cylindrospermopsins), neurotoxins (such as anatoxins and saxitoxins) and dermatoxins.
Microcystins
Microcystins (MCYST) are hepatotoxic cyanobacterial toxins. In early 1959, the first microcystins were isolated from the cyanobacterium Microcystis aeruginosa as so called “death factor”. The isolated compounds were later named microcystin after the source organism Microcystis aeruginosa.
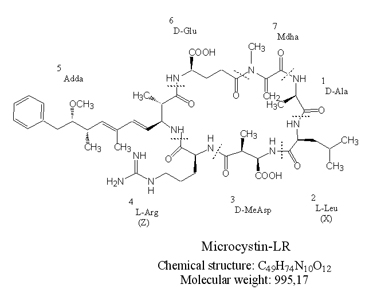
Structure
In the early 1980s the complete structure of the first microcystin variant microcystin-LA was identified. Microcystins are cyclic heptapeptides with the general structure: cyclo-(D-alanine1-X2-D-MeAsp3-Z4-Adda5-D-glutamate6-Mdha7). The amino acids in position 2 and 4 are variable L amino acids, D-MeAsp3 is D-erythro-β-methylasparticacid, and Mdha is N-methyldehydroalanine. The amino acid Adda, is (2S,3S,8S,9S)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid. Structural variations have been found in all seven amino acids. The most frequent variations are substitutions of the L-amino acids at position 2 and 4 and demethylation of amino acids at positions 3 and 7. Today more than 80 different microcystins are known.
Source organisms
Microcystins are produced by cyanobacteria belonging to different genera e.g. Microcystis, Anabaena, Oscillatoria, Planktothrix, and Nostoc. They are most commonly found in cyanobacterial blooms in freshwater worldwide. Marine sources for microcystins are not yet known.


Microcystis aeruginosa
Toxicity
Microcystins are synthesized and are mostly retained in the living cyanobacterial cells. After senescence and cell lysis they are released into the surrounding water. Due to their chemical stability microcystins can persist in the water for several days or weeks after the breakdown of the cyanobacterial bloom. Most Microcystins are water soluble and can not penetrate lipid membranes of humans, animals and plants. However, microcystins have been implicated in poisonings of humans and animals. After oral uptake microcystins are transported, particularly through bile acid-type transporters, from the ileum into the bloodstream and finally are concentrated in the liver as a result of active uptake by hepatocytes. Microcystins are primarily hepatotoxins. Acute exposure to microcystin causes severe liver damage. Characteristic features are a disruption of liver cell structure (due to damage to the cytoskeleton), a loss of sinusoidal structure, increase in liver weight due to intrahepatic haemorrhage, haemodynamic shock, heart failure and death. Other organs affected by microcystins are kidneys, intestines and lungs. Microcystins are liver tumor promoters mediated through inhibition of protein phosphatase type 1 and type 2A activities. One of the most toxic microcystin variants is microcystin-LR which has an LD50=50 µg kg-1 (mouse, intraperitoneal). Orally applied Microcystis extracts were found to have an around 50- to 170-fold higher LD50. The World Health Organization (WHO) has established a guideline of 1 μg/l as a maximum concentration of microcystin-LR in drinking water.
References
Chorus, I. and Bartram, J. (1999) Toxic Cyanobacteria in Water: a Guide to Public Health Significance, Monitoring and Management. E & FN Spon /Chapman & Hall, London, 416 pp.
http://www.who.int/water_sanitation_health/resourcesquality/toxicyanbact/en/
Welker, M. and Döhren, H. (2006): Cyanobacterial peptides – Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 30:530–563.
WHO (2003) Cyanobacterial toxins: Microcystin-LR in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/57).
WHO (2011) Guidelines for drinking-water quality – 4th ed. Geneva, World Health Organization. ISBN 978 92 4 154815 1
https://www.unicef.org/cholera/Chapter_4_prevention/01_WHO_Guidelines_for_drinking_water_quality.pdf
Nodularins
Nodularins (NOD) are hepatotoxic cyanobacterial toxins.
The first animal poisonings described in literature caused by Nodularin producing cyanobacterium Nodularia spumigena was described alredy in 1878 from Australia.
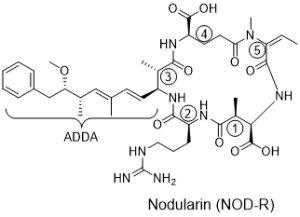
Structure
Nodularins are cyclic pentapeptides with the general structure: cyclo-(D-MeAsp1-Z2-Adda3-D-Glu4-Mdhb5).The structure of Nodularin was first identified in 1988, although the first poisoning from this was described in 1878. Today 8 different nodularins are known.
Source organism
Nodularin is produced by cyanobacteria of the genus Nodularia which is a common genus in brackish water bodies.
Toxicity
Nodularins have been implicated in poisonings of humans and animals. Nodularin has an LD50=50–60 µg kg-1 (rat, intraperitoneal).
Cylindrospermopsins
Cylindrospermopsins (CYN) are hepatotoxic cyanobacterial toxins.
The toxin was discovered after an poisoning episode in 1979 in Australia, where 138 children and 10 young adults got sick. The symptoms were malaise, vomiting, anorexia, enlarged livers, bloody diarrhea and hematuria. The incidence was known as the “Palm Island mystery disease” was connected to a local drinking water source experiencing a heavy algal bloom treated with copper sulfate five days earlier.
Anatoxins
Anatoxin-a (ANTX) is a neurotoxic cyanobacterial toxin. Already in the 1930´s cyanobacterial blooms were observed causing very fast deaths in animals. The causing agents were therefore called “very fast death factor (VFDF)”. The toxin was later identified and named anatoxin-a. It was the first cyanobacterial toxin which was chemically and functionally defined in 1972.
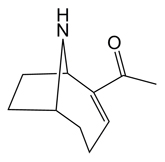
Structure
Anatoxin-a is a bicyclic secondary amine with the general structure 2,9-Diacetyl-9-azabicyclo[4,2,1]non-2,3-ene. Besides ANTX, homoanatoxin-a a methylated anatoxin-a analogue , and 4-hydroxyanatoxin-a an oxygenated homoanatoxin-a analogue, have been isolated and described.
Source organisms
Anatoxin–a was first isolated and identified from the cyanobacterium Anabaena flos-aquae in 1972. Anatoxin-a was later found in various members of the cyanobacterial genera Anabaena, Aphanizomenon, Cylindrospermum, Oscillatoria, Planktothrix and Raphidiopsis. Homoanatoxin-a was first detected in Oscillatoria sp. in 1992 and later found to be also produced by members of the cyanobacterial genera Anabaena, Raphidiopsis and Phormidium. 4-hydroxyanatoxin-a was isolated from a Rhaphidiopsis strain in 2003.
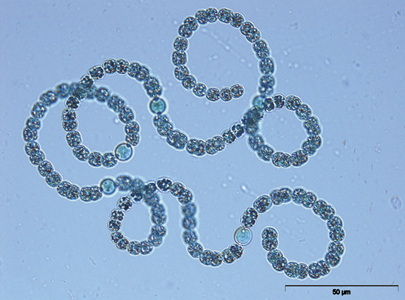
Light microscopy photo of Anabaena flos-aquae
Toxicity
Anatoxin-a is a potent agonist of the muscular and neuronal nicotinic acetylcholine receptor. Anatoxin-a is a structural analogue to the neurotransmitter acetylcholine and binds to the nicotinic acetylcholine receptor with a higher affinity than acetylcholine. It acts as a postsynaptic depolarizing neuromuscular blocking agent and cannot be degraded by acetylcholinesterase thus causing muscle overstimulation. Signs of poisoning are staggering, muscle fasciculations leading to fatigue and paralysis, gasping, convulsions, and death by respiratory arrest in vertebrates. Homoantoxin-a is a potent analogue of anatoxin-a. Anatoxin-a and homoanatoxin-a are highly toxic with a LD50=200-250 µg kg-1 (mouse, intraperitoneal). 4-Hydroxyanatoxin-a does not show apparent toxicity to mice.
References
Aráoz R., Molgó J., Nicole Tandeau de Marsac, N. (2010): Neurotoxic cyanobacterial toxins. Toxicon 56, 813–828.
Chorus, I. and Bartram, J. (1999) Toxic Cyanobacteria in Water: a Guide to Public Health Significance, Monitoring and Management. E & FN Spon /Chapman & Hall, London, 416 pp.
http://www.who.int/water_sanitation_health/resourcesquality/toxicyanbact/en/
Namikoshi, M., Murakamia, T., Watanabe, M.F., Yamada, T.O. J., Tsujimura, S, Nagaia, H., Oishie, S. (2003): Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 42, 533–538.