Is P-glycoprotein involved in toxicity of deoxynivalenol?
4-Deoxynivalenol (DON) is the most prevalent Fusarium mycotoxin, and it frequently occurs in grains such as wheat, barley, oats and rye all over the world. The ingestion of DON contaminated feed has been shown to result in pro-inflammatory effects as well as various pathological lesions, including the necrosis of the intestinal epithelium in several animal species. Furthermore, several in vitro and in vivo studies have demonstrated that DON compromises the intestinal barrier function. The underlying mechanisms of DON toxicity and a potential connection with intestinal disorders in humans and animals are still unclear.
The aim of the present study was to further characterise the nature of DON interactions with Pgp in a number of test systems including human colorectal adenocarcinoma (Caco-2) cells and Madine-Darby canine kidney cells, wild-type (MDCKII-wt) and Pgp-expressing (MDCKII-MDR1). Lysosomal activities in MDCKII-wt and MDCKII-MDR1 cells were assessed using the Neutral Red assay. DON-induced changes in cytosolic pH of MDCKII-wt cells were monitored using BCECF-AM. Several known Pgp-inhibitors (e.g.: verapamil (Ver, 100 μM) and valspodar (PSC 833, 1 μM)) were used in the Pgp inhibition assays utilising fluorescent Pgp substrate, such as calcein acetoxymethyl ester (calcein AM, 1 μM). Results were further evaluated by performing an additional set of experiments with verrucarol, a structural analogue of DON lacking the C-8 ketone function in order to test the importance of the α,β -unsaturated ketone moiety for activity towards Pgp.
Bidirectional transport studies performed in Caco-2 cells demonstrated that the uptake of DON was 1.1±0.1 times (±SD, n=5, 2h) higher in the BL→AP direction as compared to the opposite direction, indicating efflux carrier-mediated active transport of DON (Table 1). Furthermore, MDCKII-MDR1 cells showed significantly higher tolerance to DON as compared to MDCKII-wt cells in the Neutral Red cytotoxicity assay (IC50 MDCKII-wt 3.1±0.3 µM vs IC50 MDCKII-MDR1 6.7±0.6 µM), confirming the involvement of Pgp in the transport of DON. A significant decrease in cytosolic pH was demonstrated in MDCKII-wt cells (DON 2.5 and 5 µM, 24h) highlighting the role of Pgp in DON-mediated in vitro toxicity.
IC50 in µM; a=statistically significant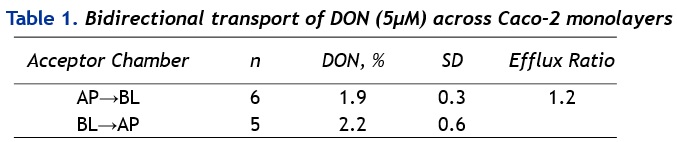
In contrast to DON, the DON-analogue verrucarol has demonstrated similar toxicity in MDCKII-wt and MDCKII-MDR1 cells, highlighting the importance of the α, β-unsaturated carbonyl function for the binding of DON to human Pgp. This assumption was supported by further comparing the effects of both compounds in the Calcein AM assay in MDCKII-MDR1 cells, where DON acted as potential modulator while verrucarol was significantly less potent to reduce the calcein retention achieved in the presence of selective inhibitors of Pgp (Ver, PSC 833).
Immunoblot analysis (dot blot) using a specific anti-DON antibody showed noticeable differences in the binding intensities to DON bound to cell membranes of exposed (5 µM, 24 h) MDCKII-wt and MDCKII-MDR1 cells.
In conclusion, our results support the ability of DON to interact with Pgp.