Bog asphodel (Narthecium ossifragum) is a perennial herb, 5 – 40 cm tall, with a creeping rhizome. It has two rows of grass-like leaves, flattened into a sheath at ground level, and a slender raceme of bright yellow flowers approximately 1 cm in diameter. The plant occurs on oligotrophic, mesotrophic and eutrophic peat deposits in Scandinavia, in the British isles, in the Netherlands, Belgium, Northwest Germany, Western and Central France, Northern Spain and East Portugal. In Norway the plant is most common on bogs and heathery moors which are low in calcium, on the western coast up to Troms County and up to Ringerike in eastern Norway. Grazing of the plant has for several hundreds years been associated with osteomalacia in cattle, therefore the species name ‘ossifragum’. The plant grows on calcium deficient ground and therefore probably has low calcium content. However, the reason for the observed osteomalacia in cattle grazing N. ossifragum may as well be the overall low calcium content in the pasture plants in these areas.
Hepato- and nephrotoxicity related to Narthecium ossifragum
N. ossifragum has for several hundred years been associated with the hepatogenous photosensitivity disease alveld. Photosensitization of sheep grazing N. ossifragum-containing pastures is also reported to occur in the Faroe Islands and the British Isles. Occasionally in Norway, 30-50 % of the lambs in certain flocks grazing N. ossifragum will suffer from alveld, so the disease is of economic importance as well as a welfare problem. Normally, the disease occurs only in lambs 2-6 months of age, and more cases are seen in cold and rainy summers than in hot and dry summers. Breed differences in susceptibility to the disease has been demonstrated. Hepatogenous photosensitization occurs when a toxin, normally produced by a higher plant, a fungus or an alga, causes liver damage or liver dysfunction that result in retention of the photosensitizing agent, phylloerytrin. Phylloerythrin is a metabolic product of chlorophyll produced by rumen microorganisms, and an acute inflammatory response of the skin is induced when phylloerythrin reacts with sunlight. Similar photosensitivity diseases occur when sheep graze Agave lecheguilla, Brachiaria decumbens, Panicum spp. and Tribulus terrestris. All the plants involved in this group of diseases contain steroidal saponins, and it has been suggested that the saponins cause liver damage resulting in retention of phylloerythrin. However, several dosing experiments with saponins have failed to reproduce liver lesions. All photosensitization diseases associated with grazing of plants containing steroidal saponins occur sporadically, and the occurrence varies between regions, and also from year to year at a single site.
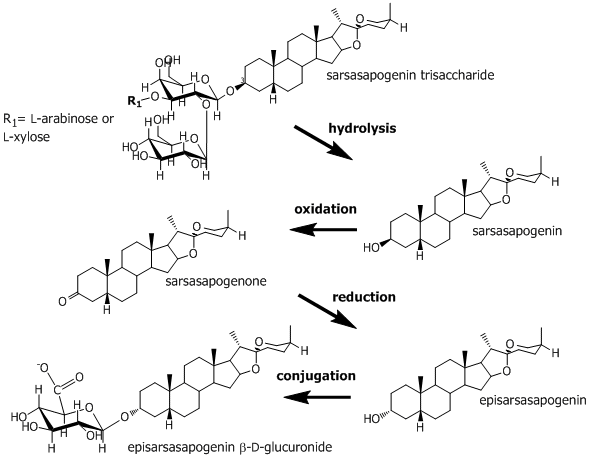
Whether the saponins are the sole cause of these photosensitization diseases is still debated, but all the diseases are characterized by the presence of calcium salts of episapogenin conjugates in the liver and in the bile ducts. The saponins from N. ossifragum are predominantly hydrolyzed in the rumen to afford free sapogenins, and a part of the free sapogenins are oxidized at C-3 and then reduced, to afford the epi-analogues of the parent sapogenins. The episapogenins are conjugated with glucuronic acid in the liver and excreted with the bile (Figure 1).
Figure 1. Proposed pathway for the ovine metabolism of sarsasapogenin-trisaccharide, the major saponin of extracts from Narthecium ossifragum
Traditionally, hepatogenous photosensitivity diseases of sheep have been divided into two groups, depending on whether the parenchyma or the biliary system is primarily affected. Kellerman and colleagues (1988) suggested that the hepatogenous photosensitization associated with Tribulus terrestris (geeldikkop) results from physical occlusion of the bile ducts and ductules by crystals, so causing retention of phylloerythrin. This hypothesis has been supported by results from several studies of geeldikkop and intoxication with Panicum sp. The suggestion that phylloerythrin retention in sheep grazing saponin-containing plants is primarily caused by obstruction of bile ducts is contradictory to results from studies on alveld, which have indicated that the hepatocellular damage is more severe than the biliary changes. Normally there are no macroscopic lesions in the livers of lambs suffering from alveld, except for the yellow discoloration due to jaundice that can be seen in some cases. The histopathological changes are dominated by single cell necrosis of hepatocytes, vacuolization of hepatocytes, portal fibroplasia and bile duct proliferation. The presence of birefringent sapogenin crystals is a typical finding. The morphological hepatocellular damage are usually not classified as severe. Hence it is suggested that the phylloerythrin retention in alveld can be caused by a dysfunction in the hepatocyte metabolism and/or dysfunctional transmembrane transport mechanisms. The presence of high concentrations of conjugated bilirubin and conjugated episapogenins in the livers of alveld-affected lambs seen in one study supports this suggestion. The pathogenesis of alveld is still not compeletely explained. It is obvious, however, that the sapogenins in some way play an important role in the aetiology of alveld, either by being the primary cause of the liver lesions or by accumulating as a result of liver damage, thereby exacerbating the liver dysfunction and retention of phylloerythrin.
In 1989 several cases of severe bovine disease related to ingestion of bog asphodel (Narthecium ossifragum) were reported in Ireland, and in 1992 similar cases were reported in cattle on pastures in Norway. Clinical signs were depression, anorexia, melaena or fresh blood in the feces, and often diarrhea, leading to death in many cases. Serum urea and creatinine concentrations were elevated, and post mortem examination revealed renal tubular necrosis. N. ossifragum was found in all pastures examined for toxic plants and mushrooms, and it was suggested to be the cause of this disease. Nine cases of lethal toxic nephrosis in moose were reported from Aust Agder County in Norway during the summers of 1995/96. Five of nine cases occurred in the second half of June. The cause of these poisoning cases was proposed to be either oak (Quercus sp.) or N. ossifragum. An experimental study confirmed the ability of N. ossifragum to cause toxic nephrosis in moose. Another study, although in sheep, indicated that fully grown oak leaves are not very toxic to ruminants. The reported number of cases of naturally occurring toxic nephrosis in moose due to N. ossifragum is low. Not all sick or dead animals are found, and the lack of cases in late summer may indicate development of tolerance to the toxic principle or the fact that the animals find other plants to graze.
Field cases of toxic nephrosis caused by ingestion of N. ossifragum have only been seen in cattle and moose. Both young and adult animals are affected. N.ossifragum poisoning is probably not a new disease in Norway, as many district veterinary officers have seen similar cases before 1992. Experimental studies have confirmed the nephrotoxic effect of N. ossifragum in cattle, sheep, goats and moose, red deer and reindeer. The clinical pathology and histopathology results were similar in all experiments. All the species mentioned above responded with elevated serum concentrations of creatinine, urea and magnesium shortly after dosing with 15 g wet weight N. ossifragum pr kg live weight (calves, lambs) or water extract from 30 g plant material pr kg live weight (goat, moose, red deer, rein deer). Serum calcium concentrations were decreased. The main histopathological findings were degeneration and necrosis of tubular epithelium in proximal straight tubules, and eosinophilic material in the tubular lumina. The nephrotoxic principle seemed to be potent and fast acting both in calves and goats. Lambs ingesting even higher doses than calves developed less severe kidney lesions, and they did not develop aversion against the N. ossifragum containing feed as calves did. The calves did not ingest all of the N. ossifragum they were offered due to reduced appetite and feed aversion already on the second day of N. ossifragum feeding. The feed aversion may be due to the rapid toxic effect on the kidneys.
In a feeding experiment, it was shown that feeding N. ossifragum to lambs in gradually increasing doses for 10-15 days, resulted in increased tolerance to the nephrotoxin. It is possible that other ruminants grazing N. ossifragum also develop tolerance to the nephrotoxin. The threshold dose for developing toxic nephrosis may vary with different species. This could explain why field cases only have been reported in some of the species known to graze N. ossifragum. In all the experimental animals there were signs of regeneration in the kidneys after a few days. The serum concentrations of creatinine, urea and magnesium decreased and in the kidney specimens there were hypertrophy of tubular epithelial cells and incipient interstitial fibroblast proliferation. No pathological changes were observed in other examined organs.
Phytochemicals with reported toxic effects in Narthecium ossifragum
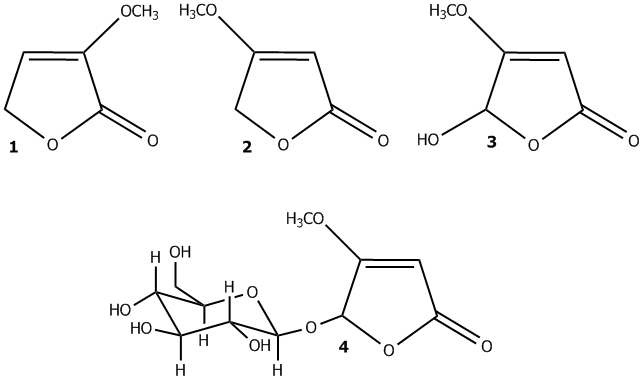
Cattle are known to be unwilling to eat N. ossifragum because the plant is toxic. However, both cattle, sheep, goats and horses graze on the plant although it is not the most preferred plant on the pastures. For many decades acute poisoning cases with anorexia, diarrhea, melena, dehydration and deaths have been observed in cattle grazing N. ossifragum in Denmark. In Norway, the plant was associated with the hepatogenous photosensitization disease “alveld” until 1992, when it was discovered that it could cause nephrotoxicity in several ruminant species as well. A great deal of research has been carried out to investigate the toxic compounds in N. ossifragum and also to study the effect of these substances both in vivo and in vitro. The most important and the best described compounds are the steroidal saponins and the furanones (Figure 2). The plant also contains a high variety of carotenoids and different sterols.
Figure 2. The furanones of Narthecium: 3-methoxyfuran-2(5H)-one (1), 4-methoxyfuran-2(5H)-one (2), 5-hydroxy-4-methoxyfuran-2(5H)-one (3), 4-methoxyfuran-2(5H)-one 5-(β-D-glucoside) (4)
Reference:
Wisløff, H. “Toxic effects of Narthecium ossifragum with emphasis on kidney lesions.” Thesis for the degree of Dr. med. vet., Norwegian School of Veterinary Science, 2008.