PARC is a very large EU-funded project with almost 200 partners in 28 countries running in the period 2021 – 2027. The project is coordinated by ANSES, the French Agency for Food, Environmental and Occupational Health & Safety. PARC has a total budget of 400 million Euro and is funded by EU Horizon 2020 programme with a 50% own contribution from all partners.
Read more about PARC on the official website here.
The main aims of PARC are to develop the scientific skills needed to address current and future challenges in chemical safety, to provide new data, methods and innovative tools for risk assessment of chemical exposure and to strengthen networks that bring together actors specialised in the different scientific fields contributing to risk assessment.
Based on our long-standing experience with mycotoxins, a main focus of the Toxinology research group at NVI in PARC is to establish new alternative methods for studies of the inflammatory responses of priority mycotoxins in the gut, their effects on the gut health and on metabolism of kinetics of the same priority compounds.
Our work is an essential part of PARC’s workpackage 5 on Hazard Assessment. WP5 will generate data to fill gaps on poorly characterised contaminant or potential emerging hazards and develop and promote the regulatory acceptance of new approaches and methods for hazard assessment.
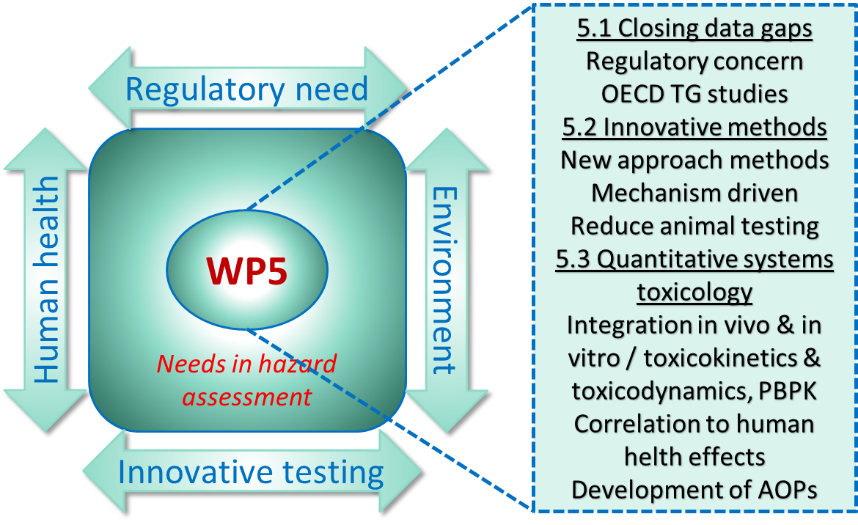
Task 5.1, we contribute to a project aiming to close data gaps on Enniatins (> 4 different toxins), Beauvericin and Alternaria toxins (> 7 different toxins), which are emerging mycotoxins with limited toxicological data. The available data are insufficient for a comprehensive risk assessment. By performing toxicity studies in different human cell lines, the toxic potentials of the toxins are evaluated. In in vitro biotransformation studies using metabolic active hepatic microsomes of humans and rats, metabolism and detoxification pathways are determined and metabolite libraries developed. Toxins and metabolites are analysed with high-resolution mass spectrometry.
In Task 5.3, we are involved in a project for the determination of the pharmacokinetic parameters of the enniatins B and B1. In close cooperation with the ANSES FOUGERES laboratory, physiology-based kinetic modeling is performed based on the integration of in vitro ADME data produced with primary hepatocytes of different species and comparison to in vivo rat toxicokinetic studies.
Publications
- Louro H, Vettorazzi A, López de Cerain A, Spyropoulou A, Solhaug A, Straumfors A, Behr A-C, Mertens B, Žegura B, Fæste CK, Ndiaye D, Spilioti E, Varga E, Dubreil E, Borsos E, Crudo F, Eriksen GS, Snapkow I, Henri J, Sanders J, Machera K, Gaté L, Le Hegarat L, Novak M, Smith NM, Krapf S, Hager S, Fessard V, Kohl Y, Silva MJ, Dirven H, Dietrich J, Marko D. Hazard characterization of Alternaria toxins to identify data gaps and improve risk assessment for human health. Archives of Toxicology 2024, 98, 425-469.
- Snapkow, I., Smith, N., Arnesdotter, E., Beekmann, K., Blanc, E. B., Braeuning, A., Corsini, E., Dolenc, M.S., Duivenvoorde, L.P.M., Eriksen, G.S., Franko, N., Galbiati, V., Gostner, J.M., Grova, N., Gutleb, A.C., Hargitai, R., Janssen, A.W.F., Krapf, S.A, Lindeman, B., Lumniczky, K., Maddalon, A., Mollerup, S., Parráková, L., Pierzchalski, A., Pieters, R.H.H., Silva, M.J., Solhaug, A., Staal, Y.C.M., Straumfors, A., Szatmári, T., Turner, J.D., Vandebriel, R.J., Zenclussen, A.C., Barouki, R. New approach methodologies to enhance human health risk assessment of immunotoxic properties of chemicals—a PARC (Partnership for the Assessment of Risk from Chemicals) project. Frontiers in Toxicology 2024, 6, 1339104.
Presentations
- Solhaug A, Jakaviciute R, Eriksen GS. Effects of enniatins and Alternaria mycotoxins on a human intestinal model. PARC WP5 Annual Meeting, 27.-29.5.2024, Lisbon, Portugal
- Deepika D, Fæste CK, Sylvia E, Kumar V. New Approach Methodologies (NAMs) for risk assessment in the context of physiologically based kinetic and quantitative systems toxicology models. SETAC Europe 34th Meeting, 5.-9.5.2024, Seville, Spain.
- Ivanova I, Jespers V, Rangel-Huerta OD, Fæste CK. Accelerating the identification of enniatins and related metabolites. PARC WP5 Annual Meeting, 27.-29.5.2024, Lisbon, Portugal
- Dubreil E, Ivanova L, Gendre C, Madjhoub M, Fessard V, Le Hegarat L, Fæste CK, Henri J. Metabolism of enniatin B in primary mouse, rat and human hepatocytes. EUROTOX-2024, 8.-11.9.2024, Copenhagen, Danmark