 Hen’s (Gallus domesticus) egg is an important component of human nutrition, and at the same time one of the most common food allergens. Many industrialised countries have therefore introduced mandatory labelling of egg in food products. The prevalence of egg allergy (1.6 %) is highest in children aged 2.5 years and decreases in adults because of the development of tolerance. Among children diagnosed with atopic dermatitis, about 35 – 45 % have been found to be allergic to egg, whereas about 12 % of the food-allergic adults react to egg.
Hen’s (Gallus domesticus) egg is an important component of human nutrition, and at the same time one of the most common food allergens. Many industrialised countries have therefore introduced mandatory labelling of egg in food products. The prevalence of egg allergy (1.6 %) is highest in children aged 2.5 years and decreases in adults because of the development of tolerance. Among children diagnosed with atopic dermatitis, about 35 – 45 % have been found to be allergic to egg, whereas about 12 % of the food-allergic adults react to egg.
The range for the lowest provoking doses was found at 1 mg to 400 mg egg-containing food (0.13 – 200 mg egg protein) by double blind placebo-controlled food challenge (DBPCFC) studies. However, an allergic reaction after the intake of only 0.03 mg spray dried whole egg has also been described.
Egg allergens
Egg white contains approximately 40 different proteins. The most important allergens in the egg white are ovomucoid (OM) (Gal d 1; 11 % of the total protein content), ovalbumin (Gal d 2; 54 %), ovotransferrin (OT) (Gal d 3; 12 %), and lysozyme (LY) (Gal d 4;3.5 %). They are all glycoproteins. Additionally, serum albumin (Gal d 5) is of certain importance. The egg yolk contains the minor allergens phosvitin (Gal d 6), and apovitellin VI (Gal d Apo VI).
OM is a 28 kDa protein with trypsin-inhibitor activity containing 186 amino acids arranged in three tandem domains with internal disulfide bonds and has a carbohydrate moiety of about 30 %. Under food processing procedures like stirring, heating or enzymatic hydrolysis the protein’s IgE-binding epitopes remain relatively stable so that the allergenic potential is preserved. When heated in the presence of wheat flour, OM becomes insoluble by forming intermolecular disulfide protein-polysaccharide interactions, making the molecule less extractable.
OA is a 45 kDa protein containing 385 amino acids and at least five IgE-binding epitopes. The allergenicity of the thermolabile molecule diminishes by heating in solution. OT is a 77.7 kDa iron-binding protein with 686 amino acids and has reduced IgE-binding capacity after heat treatment. In the presence of milk (alpha casein protein) the heat-induced aggregation of OT can be suppressed due to interaction of phosphoserine residues leading to the forming of a transparent egg white gel. LY is a 14.3 kDa protein with 129 amino acids. Although its allergenicity is reduced by heat treatment, the use of the bacteriolytic LY as a food preservative in processed foods means a certain risk for egg-allergic consumers.
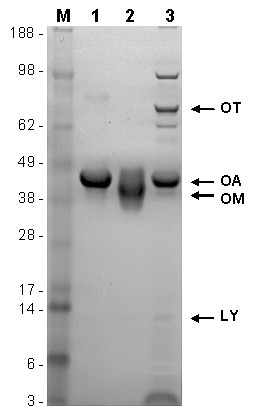
Figure 1: SDS-PAGE gel of egg proteins: M: molecular weight marker (kDa), ovalbumin (1), ovomucoid (2), and spray-dried egg white powder (3). The positions of OT, OA, OM, and LY are indicated.
Detection of egg proteins in processed foods
Egg proteins are structurally altered by food processing methods and can bind permanently to e.g. wheat and milk proteins. Therefore, the detectability of egg proteins in processed foods by ELISA is dependent on efficient extraction from the food matrix and the quality of the used anti-egg antibody, which has to detect also modified egg proteins.
We have tested the performance of three commercially available kits for quantitative egg analysis using six model heat-processed foods. The three assays worked well under standard conditions with soluble egg white proteins, however only the kit using a denaturing-reducing extraction buffer detected egg in complex heat-treated food matrices.
See our publication in the Journal of the AOAC International here.