Fish has always been an important source of dietary protein, and it is a main component of the diet especially in coastal areas. Edible fish include more than 20,000 species; although the most commonly consumed ones belong to only a few orders of the ray-finned fish (Actinopterygii). The world average fish consumption per year and consumer has constantly been increasing in the last years and amounts now to 16 kg. Further overall growth is expected if the demand for fish increases in countries with presently low fish consumption. To meet consumer demands, the fish industry has been expanding through the proliferation of fish farms and fish processing industries. A large share of fish is now consumed not longer as entity but fish and its derivatives are also used as ingredients in processed foods and dietary supplements such as fish gelatine, fish oil and omega-3 fatty acids.


Rock carvings dated to 4200 BC at Alta, Norway (UNESCO World Heritage Site)
Allergy to fish represents one of the most prevalent causes for severe food-allergic reactions. It has been described as early as 1921 in a classical study by Prausnitz and Küstner, which was pioneering in the history of allergology. Fish belongs to the four types of food that most often provoke a serious anaphylactic reaction, and is especially significant in populations where fish is a major component in the food. The prevalence of fish allergy is estimated to 0.1 % – 0.3 % of the total population in industrialized countries. Other clinical manifestations in fish allergic patients except for anaphylaxis are urticaria and/or angioedema after ingestion of fish, or asthma after inhalation of airborne fish allergen.
A few mg of fish can be sufficient to provoke allergic reactions in individuals with fish hypersensitivity. The minimum amounts needed to elicit symptoms were reported in the range between 5 to 6000 mg of fish. Since doses relate to the total fish and not to fish protein, threshold doses given in mg protein are even lower, approximately 1 to 100 mg protein, considering that fish has a total protein content of 15 to 25 %.
Regulatory authorities in many countries have implemented legislations that mandate the labelling of allergenic foods and products thereof when they are use as an ingredient. This includes all fish species in any form or state of food processing, e.g. boiled, fried, dried, salted, fermented or raw, and products like fish oils, gelatine or hydrolysates. However, fish gelatine and the finishing agent isinglass, two highly purified and degraded products, have been exempted from these regulations.
Fish allergens – the ubiquitous allergen parvalbumin
Fish allergens belong to the collective allergens and are considered as a group including many species that contain homologues of one dominating allergenic protein. The major allergen in fish is parvalbumin, a protein of 12 kDa containing 108-109 amino acid residues. The abundant fish parvalbumin is among the first food allergens that have been isolated, crystallised and identified on a molecular level. Parvalbumin is a sarcoplasmatic muscle protein. It is well conserved, highly water-soluble and resistant to heat, denaturing agents and extreme pH. Parvalbumin contains three EF-hand motifs with two high-affinity calcium ion-binding sites. The protein has a function in calcium buffering and is probably involved in muscle relaxation. If bound calcium is removed, the allergenicity of the protein diminishes. Parvalbumins are found at great amounts in the fast skeletal muscles of low vertebrates like fish and frog. The content can be as high as 2g /kg fish muscle. Only fish and frog parvalbumins are confirmed allergens, belonging phylogenitically to the β-lineage of this protein family, which is divided into two evolutionary distinct subfamilies. In contrast, the parvalbumins in the fast twitch muscle of higher vertebrates are of α-lineage and apparently non-allergenic.
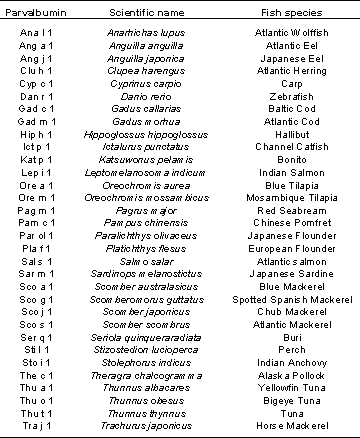
Fish parvalbumins are considered to be the major and sole allergens for 95 % of patients suffering from IgE-mediated fish allergy. The protein contains at least five IgE binding sequential and conformational epitopes. Additionally, about 15 minor fish allergens with molecular weights ranging from 15 to 200 kDa have been identified. Parvalbumins from more than 50 different fish species, among them several from commercially used fish, have been identified and molecularly characterised by now (see table below).
Development of a quantitative sandwich ELISA for the detection of fish in foods
Avoidance of fish-containing foods is the only precaution for fish-allergic consumers. During food manufacturing processes and the preparation of meals, great care is required to avoid cross-contamination. Analytical methods for the detection of fish in foods are necessary to monitor hygiene practices and to allow the enforcement of legislation.
 Detection of fish proteins in food has previously been based on the use of patient serum. A novel sandwich enzyme-linked immunosorbent assay (ELISA) for the quantitation of fish in food matrixes has been developed in our laboratory, using polyclonal rabbit anti-cod (Gadus morhua) parvalbumin antibodies for capture and a biotinylated conjugate of the same antibodies for detection. By employing the ubiquitous muscle protein parvalbumin as target the method succeeds to detect a variety of fish, although with different recoveries. However, the ELISA is specific for fish and does not cross-react
Detection of fish proteins in food has previously been based on the use of patient serum. A novel sandwich enzyme-linked immunosorbent assay (ELISA) for the quantitation of fish in food matrixes has been developed in our laboratory, using polyclonal rabbit anti-cod (Gadus morhua) parvalbumin antibodies for capture and a biotinylated conjugate of the same antibodies for detection. By employing the ubiquitous muscle protein parvalbumin as target the method succeeds to detect a variety of fish, although with different recoveries. However, the ELISA is specific for fish and does not cross-react

with other species. Recoveries ranged from 68 – 138 % in typical food matrixes, while the intra- and inter-assay precisions were < 12% and < 19 %, respectively. The cod parvalbumin ELISA has a limit of detection of 0.01 mg parvalbumin/kg food corresponding to about 5 mg fish/kg food.
See our publication in the Journal of Immunological Methods here
“Broad-spectrum” detection of fish species by “multi-fish” analysis
The detection of multiple fish species in food was improved by generating a polyclonal antiserum to six commonly consumed fish species (cod, salmon, mackerel, herring, bluefin tuna, and sprat). The ”multi-fish” approach to immunisation may be a viable alternative in the development of ELISA methods with a broader application in detecting fish protein in foods.

Monoclonal antibody to fish parvalbumin
An IgG monoclonal antibody against cod parvalbumin was produced in mice. The MoAb has cross-reactivities against parvalbumin from different fish species. A competitive ELISA for the detection of fish parvalbumin was set up and dose-dependent inhibition was found.
Immunogenicity of fish proteins in processed foods
Food processing procedures like boiling, drying, fermenting and canning can alter the antigenicity and allergenicity of fish allergens. However, the major fish allergen parvalbumin of cod remained allergenic even after heating at 100°C, digestion with proteolytic enzymes or denaturation with chemicals. In a study on clinical reactivity of ten fish species after boiling, allergenicity was not eliminated. In contrast, canning appeared to reduce allergenic reactions to tuna and salmon, probably because manufacturing of canned products includes cooking for up to 14 h.
Fish protein immunogenicity was studied by IgE binding of sera from patients allergic to fresh and processed (smoked, salted/sugar-cured, canned, lye-treated and fermented) cod, haddock, salmon, trout, tuna, mackerel and herring and of hydrolysates from salmon and whiting using products from the Norwegian marked. Stable allergenic parvalbumin oligomers were detected in all species except tuna. Chemical processes generally caused loss in IgE-binding activity, though sensitization may occur to modified or degraded rather than intact peptides.
See our publication in International Archives of Allergy and Immunology here
MS-based detection method for fish parvalbumin
The development of a method using mass spectrometry for the detection of fish in foods was initiated by finding suitable marker peptides of parvalbumin. The protein solution was separated by capillary reversed phase HPLC and analysed by tandem mass spectrometry using an API 4000 Triple Quadrupole LC/MS/MS. Two suitable precursor ion – product ion transitions were identified:

359 m/z -> 341 m/z
397 m/z -> 113 m/z
712 m/z -> 381 m/z
712 m/z -> 352 m/z
739 m/z -> 381 m/z